Health Testing
What does it all mean?
Responsible breeders will test their dogs for genetic diseases that are known to pop up in their breed.
You will see "Clear for" or "Carrier for" x genetic disease listed on the dog's bio. It sounds, and is really great when a dog is "Clear" for any or all genetic diseases, but why exactly is it so great that they are? Why should you care?
Please note that being a carrier for (most of the) genetic diseases does not mean the dog will be affected. In most cases a dog needs to receive 2 copies of a gene (affected vs carrier) to be affected.
Some of the diseases affect your dogs ability to metabolize certain drugs (which can be fatal if carrier or affected status is not known), while others tested for don't show up until later on in life.
This testing is completed so we know we are breeding dogs who will not run into these issues, avoiding health problems, and potential heartbreak for their families.
Read on below for specific descriptions of the genetic diseases tested for in the Miniature American Shepherd
You will see "Clear for" or "Carrier for" x genetic disease listed on the dog's bio. It sounds, and is really great when a dog is "Clear" for any or all genetic diseases, but why exactly is it so great that they are? Why should you care?
Please note that being a carrier for (most of the) genetic diseases does not mean the dog will be affected. In most cases a dog needs to receive 2 copies of a gene (affected vs carrier) to be affected.
Some of the diseases affect your dogs ability to metabolize certain drugs (which can be fatal if carrier or affected status is not known), while others tested for don't show up until later on in life.
This testing is completed so we know we are breeding dogs who will not run into these issues, avoiding health problems, and potential heartbreak for their families.
Read on below for specific descriptions of the genetic diseases tested for in the Miniature American Shepherd
MDR1
Multidrug Resistance 1 is an inherited condition affecting several breeds of dogs, primarily herding dogs. The mutation causes dysfunction of P-glycoprotein, which is responsible for removing certain drugs and toxins from the body. If an at risk dog is treated with one of the several common drugs (see below), they are at risk of developing neurological symptoms that could range from tremors, excess salivation, anorexia and blindness, to coma and potentially, death. Because of the defective ability to metabolize specific drugs, these drugs can be lethal, even at low doses.
*Drugs known to cause neurological signs related to the MDR1 mutation: Acepromazine, butorphanol, doxorubicin, emodepside, erythromycin, ivermectin, loperamide, milbemycin, moxidectin, rifampin, selamectin, vinblastine and vincristine
MDR1 is Autosomal Incomplete Dominant, meaning they only need to inherit one copy of the mutated gene to be at an increased risk of the disease. Though adverse reactions to certain drugs are most commonly seen in dogs having two copies of the mutated gene, carrier dogs can also experience drug sensitivities and dosages need to be adjusted accordingly.
**Clear dogs have been known to react to some of these drugs also, so we recommend treating ALL Miniature American Shepherds as though they are MDR1 affected, even if they are clear for it.
*Drugs known to cause neurological signs related to the MDR1 mutation: Acepromazine, butorphanol, doxorubicin, emodepside, erythromycin, ivermectin, loperamide, milbemycin, moxidectin, rifampin, selamectin, vinblastine and vincristine
MDR1 is Autosomal Incomplete Dominant, meaning they only need to inherit one copy of the mutated gene to be at an increased risk of the disease. Though adverse reactions to certain drugs are most commonly seen in dogs having two copies of the mutated gene, carrier dogs can also experience drug sensitivities and dosages need to be adjusted accordingly.
**Clear dogs have been known to react to some of these drugs also, so we recommend treating ALL Miniature American Shepherds as though they are MDR1 affected, even if they are clear for it.
PRA-prcd
Progressive retinal atrophy, progressive rod-cone degeneration is a late onset inherited eye disease. PRA-prcd occurs as a result of degeneration of both rod and cone type photo-receptor cells of the retina, which are important for vision in dim and bright light. The rod type cells are affected first. Affected dogs will initially have vision deficits in dim light (night blindness) and loss of peripheral vision. Over time, affected dogs continue to lose night vision and begin to show visual deficits in bright light. Although there is an individual and breed variation in the age of onset and the rate of disease progression, it eventually progresses to complete blindness in most dogs.
PRA-PRCD is Autosomal Recessive, meaning they need to inherit two copies of the mutated gene (one from each parent) to develop the disease
PRA-PRCD is Autosomal Recessive, meaning they need to inherit two copies of the mutated gene (one from each parent) to develop the disease
DM
Degenerative Myelopathy is an inherited neurological disorder caused by a mutation of the SOD1 gene. There is a variable presentation between breeds, which suggests that there are environmental or other genetic factors responsible for modifying disease expression. The average age of onset for dogs with DM is approx. 9 years of age. Affected dogs usually present in adulthood with gradual muscle atrophy and loss of coordination, typically beginning in the hind limbs due to degeneration of the nerves. The condition is not typically painful for the dog, but will progress until the dog is no longer able to walk. Late in the progression of disease, dogs may lose fecal and urinary continence, and the forelimbs may be affected. Affected dogs may lose full ability to walk 6 months to 2 years after the onset symptoms.
DM is Autosomal Recessive, meaning they need to inherit two copies of the mutated gene (one from each parent) to develop the disease
DM is Autosomal Recessive, meaning they need to inherit two copies of the mutated gene (one from each parent) to develop the disease
HC
Hereditary Cataracts is an inherited eye disease affecting dogs. Cataracts are opacities in the lens of the eye caused by structural changes in lens proteins. A normal lens allows light to pass through the parts of the lens affected by cataracts and vision becomes blurry. Dogs with HC most commonly present between 2-7 years of age with small cataracts that are visible on a veterinary eye exam. Dogs that carry a single copy of the mutation have an increased risk over the general (normal) population of developing cataracts. In dogs that inherit one copy of the mutation, cataracts develop slowly, sometimes leading to complete blindness. It has been speculated that dogs carrying two copies of the mutation are more likely to develop a more rapidly progressing and severe cataract.
HC is Autosomal Dominant, meaning, they only need to inherit one copy of the mutated gene to be at risk for the disease
HC is Autosomal Dominant, meaning, they only need to inherit one copy of the mutated gene to be at risk for the disease
CEA
Collie Eye Anomaly (also known as choroidal hypoplasia, CH) is an inherited disease that affects several dog breeds. The choroid is the layer of tissue in the eye responsible for supplying blood and nutrients to the retina. In dogs affected with CEA, the choroid does not develop properly and is therefore thinner than normal. The severity of the condition can vary from dog to dog. In mild cases, affected dogs may only show signs of CEA on eye exam between about 5 and 12 weeks of age, just before normal age-related pigmentation of the retina, which often masks the characteristic, disease-related changes. After this time period, mildly affected dogs may be impossible to distinguish from unaffected dogs on eye exam, and may not display obvious vision deficits. In more severe cases, clinical signs include malformations of the eye and/or optic nerve (colobomas), retinal detachment, intraocular bleeding, and subsequent blindness.
CEA is Autosomal Recessive, meaning they need to inherit two copies of the mutated gene (one from each parent) to develop the disease
CEA is Autosomal Recessive, meaning they need to inherit two copies of the mutated gene (one from each parent) to develop the disease
CMR1
Multifocal Retinopathy 1 is an inherited disorder of the retina. Affected dogs typically present between 11-16 weeks of age with multiple discrete circular areas of retinal detachment with underlying fluid accumulation that are visible on an eye exam performed by a veterinarian. These blister-like lesions are typically found in both eyes and can appear gray, tan, orange or pink, and vary in number, size and location. Progression of retinal changes is usually slow, and new lesions are not noted after 6-12 months of age. Occasionally as affected dogs age, lesions appear to heal and are no longer visible on an eye exam. Generally the dog's vision is not affected, although vision loss has been observed in some cases of CMR1
CMR1 is Autosomal Recessive, meaning they need to inherit two copies of the mutated gene (one from each parent) to develop the disease
CMR1 is Autosomal Recessive, meaning they need to inherit two copies of the mutated gene (one from each parent) to develop the disease
CD
Cone degeneration is an inherited eye disease affecting dogs. Affected dogs develop day blindness and Photophobia (light sensitivity) between 8-12 weeks after birth due to degeneration of the cells in the eye called cone photoreceptors, which are responsible for vision in bright light. Affected dogs have normal vision in low light and structures of the inner eye appear normal on eye exam.
CD is Autosomal Recessive, meaning they need to inherit two copies of the mutated gene (one from each parent) to develop the disease
CD is Autosomal Recessive, meaning they need to inherit two copies of the mutated gene (one from each parent) to develop the disease
HUU
Hyperuricosuria in an inherited condition affecting many breeds of dog. The SLC2A9 gene codes for a protein that allows the kidneys to transport uric acid from the urine. Dogs with mutations in both copies of the gene are predisposed to have elevated levels of uric acid in the urine. Uric acid can form crystals and/or stones in the urinary tract, causing recurrent urinary tract inflammation, which can include: frequent urination, blood in urine, and straining to urinate. They may also have a loss of appetite, lethargy, weakness, vomiting, and pain. Urinary stones in the bladder can cause UTIs, or more seriously, blockage of the urethra. Both males and females can be affected, but more common in males due to differences in anatomy. Not all dogs with mutations in both copies of the gene will have symptoms of the disease, though they have increased uric acid excretion in the urine.
HUU is Autosomal Recessive, meaning they need to inherit two copies of the mutated gene (one from each parent) to develop the disease
HUU is Autosomal Recessive, meaning they need to inherit two copies of the mutated gene (one from each parent) to develop the disease
NCL
Neuronal Ceroid Lipofuscinosis 6 (NCL6) in a lysosomal storage disease affecting dogs. Affected dogs lack a specific enzyme necessary for normal metabolism. As a result, there is an abnormal accumulation of waste compounds primarily in the cells of the nervous system, leading to a range of nervous system disorders. Affected dogs typically present around 1.5 years of age with progressive neurologic disease. Symptoms include: loss of vision, behavioral change, anxiety, lack of muscle coordination, and abnormal gait. Affected dogs are often humanely euthanized by 2 years of age due to progression of the disease.
NCL6 is Autosomal Recessive, meaning they need to inherit two copies of the mutated gene (one from each parent) to develop the disease
NCL6 is Autosomal Recessive, meaning they need to inherit two copies of the mutated gene (one from each parent) to develop the disease
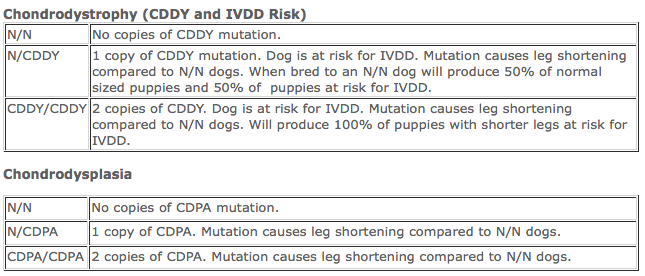
CDDY/CDPA
CDPA (Chondrodysplasia) is an FGF4-retrogene insertion in dog chromosome 18 which explains short-legged phenotype found in breeds such as Basset Hounds, Pembroke Welsh Corgis, Dachshunds, West Highland White Terriers and Scottish Terriers. CDPA inheritance is considered to follow an autosomal dominant mode, meaning, only one copy is needed to be affected (in this case, have shorter legs).
CDDY (Chondrodysdrophy) was recently discovered by researchers at the University of California, Davis, as a second FGF4-retrogene insertion in dog chromosome 12. CDDY includes a short-legged phenotype and abnormal premature degeneration of intervertebral discs leading to susceptibility to Hansen's type I intervertebral disc disease (IVDD). CDDY is inherited as a semi-dominant trait for height, meaning that dogs with 2 copies of the mutation are smaller than dogs with only 1 copy. With respect to IVDD, the inheritance follows a dominant mode, meaning 1 copy of the CDDY mutation is sufficient to predispose dogs to IVDD (**note, this does not mean they WILL get it, it just means they are more likely to**). Dogs that have both CDDY and CDPA show a more drastic reduction of leg length. How CDDY and CDPA work together to increase the risk of IVDD is still being investigated.
CDDY (Chondrodysdrophy) was recently discovered by researchers at the University of California, Davis, as a second FGF4-retrogene insertion in dog chromosome 12. CDDY includes a short-legged phenotype and abnormal premature degeneration of intervertebral discs leading to susceptibility to Hansen's type I intervertebral disc disease (IVDD). CDDY is inherited as a semi-dominant trait for height, meaning that dogs with 2 copies of the mutation are smaller than dogs with only 1 copy. With respect to IVDD, the inheritance follows a dominant mode, meaning 1 copy of the CDDY mutation is sufficient to predispose dogs to IVDD (**note, this does not mean they WILL get it, it just means they are more likely to**). Dogs that have both CDDY and CDPA show a more drastic reduction of leg length. How CDDY and CDPA work together to increase the risk of IVDD is still being investigated.
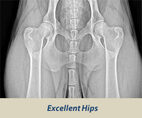
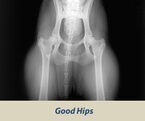
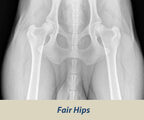
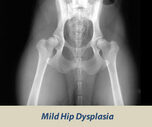
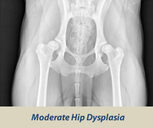
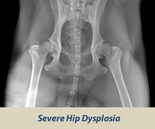
OFA HIP SCREENING
The Orthopedic Foundation for Animals (OFA) performs hip conformation evaluations on all breeds of dogs.
X-Rays are sent to the OFA, and the hip/joint conformation is graded by 3 different veterinarians into 1 of 7 classifications:
X-Rays are sent to the OFA, and the hip/joint conformation is graded by 3 different veterinarians into 1 of 7 classifications:
Passing Grades
|
|
Borderline
- Boderline: Not clear. Usually more incongruency present that what occurs in a fair but there are no arthritic changes present that definitively diagnose the hip joint being dysplasic
Dysplasic Grades
|
|
Whatever grade is given most often is what grade the dog receives.
For example: Vet #1 says Good, Vet #2 says Excellent, Vet #3 says Good, the dog will be given a Good rating.
For further information about OFA's hip rating system, click HERE
For example: Vet #1 says Good, Vet #2 says Excellent, Vet #3 says Good, the dog will be given a Good rating.
For further information about OFA's hip rating system, click HERE
OFA EYE CERTIFICATION
"The purpose of the OFA Companion Animal Eye Registry (CAER) is to provide breeders with information regarding canine eye diseases so that they may make informed breeding decisions in an effort to produce healthier dogs. CAER certifications will be performed by board certified (ACVO) veterinary ophthalmologists. Regardless of whether owners submit their CAER exam forms to the OFA for “certification,” all CAER exam data is collected for aggregate statistical purposes to provide information on trends in eye disease and breed susceptibility."
More info HERE
More info HERE